Benchmarks
View scores and output across OCR models spanning many document categories.
Want to run these evals on your own documents?
Talk to Sales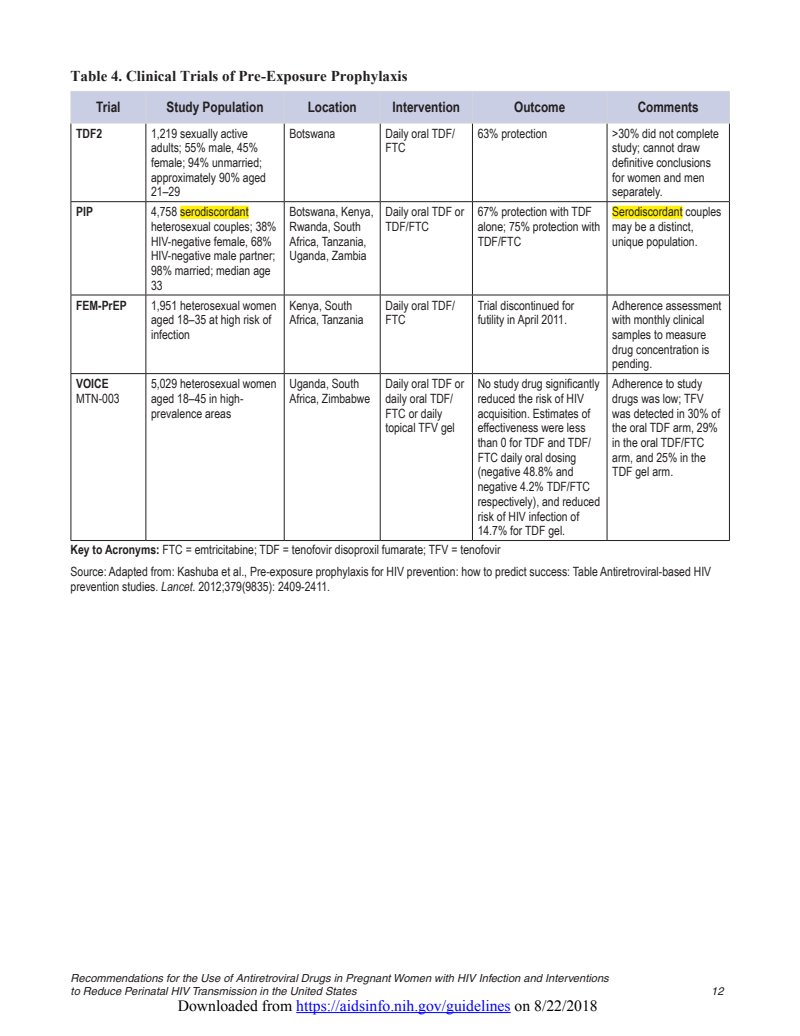
Table 4. Clinical Trials of Pre-Exposure Prophylaxis
| Trial | Study Population | Location | Intervention | Outcome | Comments |
|---|---|---|---|---|---|
| TDF2 | 1,219 sexually active adults; 55% male, 45% female; 94% unmarried; approximately 90% aged 21–29 | Botswana |
Daily oral TDF/
FTC |
63% protection | >30% did not complete study; cannot draw definitive conclusions for women and men separately. |
| PIP | 4,758 serodiscordant heterosexual couples; 38% HIV-negative female, 68% HIV-negative male partner; 98% married; median age 33 | Botswana, Kenya, Rwanda, South Africa, Tanzania, Uganda, Zambia | Daily oral TDF or TDF/FTC | 67% protection with TDF alone; 75% protection with TDF/FTC | Serodiscordant couples may be a distinct, unique population. |
| FEM-PrEP | 1,951 heterosexual women aged 18–35 at high risk of infection | Kenya, South Africa, Tanzania |
Daily oral TDF/
FTC |
Trial discontinued for futility in April 2011. | Adherence assessment with monthly clinical samples to measure drug concentration is pending. |
|
VOICE
MTN-003 |
5,029 heterosexual women aged 18–45 in high-prevalence areas | Uganda, South Africa, Zimbabwe |
Daily oral TDF or daily oral TDF/
FTC or daily topical TFV gel |
No study drug significantly reduced the risk of HIV acquisition. Estimates of effectiveness were less than 0 for TDF and TDF/ FTC daily oral dosing (negative 48.8% and negative 4.2% TDF/FTC respectively), and reduced risk of HIV infection of 14.7% for TDF gel. | Adherence to study drugs was low; TFV was detected in 30% of the oral TDF arm, 29% in the oral TDF/FTC arm, and 25% in the TDF gel arm. |
Key to Acronyms: FTC = emtricitabine; TDF = tenofovir disoproxil fumarate; TFV = tenofovir
Source: Adapted from: Kashuba et al., Pre-exposure prophylaxis for HIV prevention: how to predict success: Table Antiretroviral-based HIV prevention studies. Lancet . 2012;379(9835): 2409-2411.
Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States
Downloaded from https://aidsinfo.nih.gov/guidelines on 8/22/2018
12